Answer:
Atom having maximum mass : B
Atom which has least mass : C
Atom which is neutral : D
Identity of atom D : Lithium
Step-by-step explanation:
How to look at the Figure :
In the centre : Proton and neutrons are present
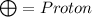
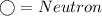
In the rings (outside the centre): electrons are present
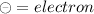
Atomic mass : Total number of proton and neutrons present in the nucleus (centre) of the atom.
To calculate atomic mass count the following symbols :
Atomic mass = Proton + neutron


Figure A
 = 4
= 4
 = 3
= 3
Atomic mass = 4 + 3 = 7
Figure B
 = 4
= 4
 = 4
= 4
Atomic mass = 4 + 3 = 8
Figure C
 = 3
= 3
 = 3
= 3
Atomic mass = 3 + 3 = 6
Figure D
 = 3
= 3
 = 4
= 4
Atomic mass = 3 + 4 = 7
Part 1 .So B has maximum atomic mass = 8 amu
Part 2. C has minimum mass = 6 amu
To check whether atoms are neutral or charged :
Count the number of protons and electrons
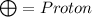 = in the centre
= in the centre
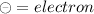 = outside the centre
= outside the centre
If the number of proton and electron are equal, then the atom is neutral
Proton = neutron
If the number of proton and electron are not equal, then the atom is Charged
Figure A. = Charged
 = 4
= 4
 = 3
= 3
Figure B = Charged
 = 4
= 4
 = 3
= 3
Figure C = Charged
 = 4
= 4
 = 2
= 2
Figure D = Neutral (equal number of proton and electron)
 = 3
= 3
 = 3
= 3
Part C .The atom which is neutral is D
Part DThe identity of Figure D : Lithium
Atomic number : Total number of proton in the nucleus of the atom
 = 3 = Proton
= 3 = Proton
So it is Lithium (See the periodic table)