Answer:
Option A.
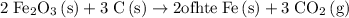 .
.
Step-by-step explanation:
Balance by the conservation of atoms
In a chemical reaction, the left-hand side of the equation should contain as many atoms of one element as the right-hand side does.
Start by assuming that the coefficient of the most complex species (e.g.,
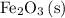 is
is
 .
.
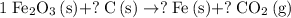 .
.
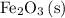 is the only species with iron and oxygen atoms on the left-hand side. On the left-hand side of the equation, it can be confirmed that there are
is the only species with iron and oxygen atoms on the left-hand side. On the left-hand side of the equation, it can be confirmed that there are
- 2 units of Fe atoms, and
- 3 units of O atoms.
By the conservation of atoms, the right-hand side should also contain that many atoms of Fe and O, respectively. Since on the right-hand side,
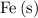 is the only species with Fe atoms, the coefficient of
is the only species with Fe atoms, the coefficient of
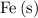 should be 2. Similarly,
should be 2. Similarly,
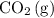 is the only species with O atoms. Additionally, each unit of
is the only species with O atoms. Additionally, each unit of
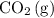 provides two units of O atoms. Hence, the coefficient of
provides two units of O atoms. Hence, the coefficient of
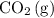 should be
should be
 .
.
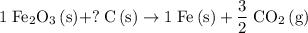 .
.
There are
 units of
units of
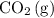 on the right-hand side. Besides, each unit of
on the right-hand side. Besides, each unit of
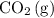 contains one C atom. Hence, it can be concluded that each side of the equation should contain
contains one C atom. Hence, it can be concluded that each side of the equation should contain
 units of C.
units of C.
All the C atoms on the left-hand side of the equation are in the form
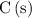 . Hence, the coefficient of
. Hence, the coefficient of
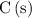 should also be
should also be
 .
.
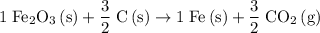 .
.
Coefficients should preferably be whole numbers. Hence, multiply all coefficients by the denominator (or the gcd of the denominators in case there are more than one of these) to get rid of the fraction. In this case, multiply all coefficients by
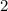 to obtain:
to obtain:
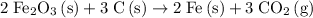 .
.