Answer:
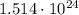
Step-by-step explanation:
Our initial goal is to convert from moles of oxygen to moles of acetaminophen. Since 1 mole of acetaminophen contains 2 moles of oxygen by stoichiometry, the amount of acetaminophen would be half of the amount of oxygen in terms of moles:
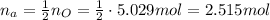
In order to convert moles to the number of molecules, we need to multiply the number of moles by the Avogadro's constant. Let's perform this calculation:
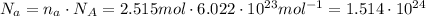
Therefore, this number corresponds to acetaminophen molecules.