Answer:
The net chemical reaction of the given process:
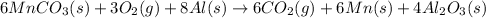
Step-by-step explanation:
Step 1: Manganese (II) carbonate and oxygen react to form manganese (IV) oxide and carbon dioxide.The chemical reaction is given as:
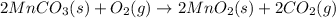
Step 2:Manganese (IV) oxide and aluminum react to form manganese and aluminum oxide.The chemical reaction is given as:
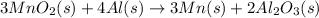
On adding reactions of 3 × [1] + 2 × [2] , we get net chemical reaction of the given process:
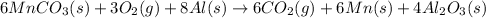
According to reaction,6 moles of manganese(II0 carbonate recats with 3 moles of oxygen gas and 8 moles of aluminum to give 6 moles of manganese , 6 moles of carbon dioxide gas and 4 moles of aluminum oxide.