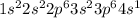Answer:
1. hydrogen - H
2. helium - He
3. sodium - Na
4. magnesium - Mg
5. potassium - K
Step-by-step explanation:
Hydrogen is the element of group 1 and first period. The atomic number of hydrogen is 1 and the symbol of the element is H.
The electronic configuration of the element hydrogen is:-
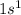
Helium is the element of group 18 and first period. The atomic number of helium is 2 and the symbol of the element is He.
The electronic configuration of the element helium is:-
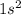
Sodium is the element of group 1 and third period. The atomic number of sodium is 11 and the symbol of the element is Na.
The electronic configuration of the element sodium is:-
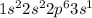
Magnesium is the element of group 2 and third period. The atomic number of magnesium is 12 and the symbol of the element is Mg.
The electronic configuration of the element magnesium is:-
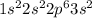
Potassium is the element of group 1 and forth period. The atomic number of potassium is 19 and the symbol of the element is K.
The electronic configuration of the element potassium is:-