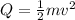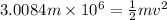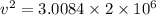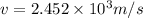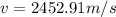Answer:
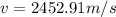
Step-by-step explanation:
Given
initially steam is at
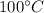 and converted to
and converted to
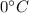 ice
ice
Let m be the mass of steam
latent heat of fusion and vaporization for water is
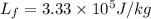
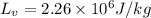
Heat required to convert steam in to water at
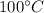
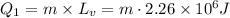
Heat required to lower water temperature to
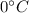
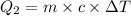
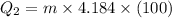
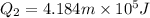
Heat required to convert
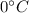 water to ice at
water to ice at
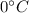 is
is
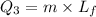
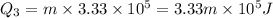
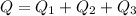
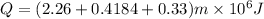
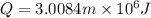
So this energy is equal to kinetic energy of bullet of mass m moving with velocity v