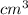1) D = 13.6 g / mL
2)ethyl alcohol weighs 158g
3)ρ _copper = 8.9 g
Step-by-step explanation:
1)
D = m / V
=306.0 g / 22.5 mL
D= 13.6 g / mL
2)
density = mass / volume
mass = density × volume
=0.789g /ml × 200.0 ml
M=158g
Ethyl alcohol weighs 158g
3)
ρ (density) = Mass / Volume
ρ _copper = 1896 g / 8.4cm × 5.5cm × 4.6cm
= 1896g / 212.5
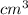
ρ _copper=8.9 g