Answer:
the light emitting must be of greater wavelength
Step-by-step explanation:
For this exercise we must use the Planck equation
E = h f
And the speed of light
c = λ f
f = c / λ
We replace
E = h c / λ
The wavelength of the green light is of the order of 500 nm, let's calculate the energy
E = 6.63 10⁻³⁴ 3 10⁸ /λ
E = 1,989 10⁻²⁵ /λ
λ = 500 nm = 500 10⁻⁹ m
E = 1,989 10⁻²⁵ / 500 10⁻⁹
E = 3,978 10⁻¹⁹ J
That is the energy of the transition for a transition is an intermediate state the energy must be less, this implies that the wavelength must increase. For the explicit case of a state with half of this energy
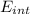 = E / 2
= E / 2
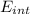 = 3,978 10⁻¹⁹ / 2 = 1,989 10⁻¹⁹
= 3,978 10⁻¹⁹ / 2 = 1,989 10⁻¹⁹
Let's clear and calculate
λ = h c / E
λ = 1,989 10⁻²⁵ / 1,989 10⁻¹⁹
λ = 1 10⁻⁶ m
Let's reduce to nm
λ = 1000 nm
This wavelength is in the infrared region
the light emitting must be of greater wavelength