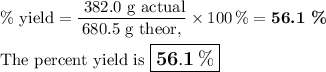Answer:
Step-by-step explanation:
We have the masses of two reactants, so this is a limiting reactant problem.
We will need a balanced equation with masses and molar masses of the compounds involved. Gather all the information in one place
Mᵣ: 64.06 32.00 80.06
2SO₂ + O₂ ⟶ 2SO₃
m/g: 544.5 160.0
1. Theoretical yield
(a) Calculate the moles of each reactant
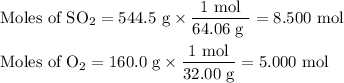
(b) Identify the limiting reactant
Calculate the moles of SO₃ we can obtain from each reactant.
From SO₂:
The molar ratio of SO₂ to SO₃ is 2:2
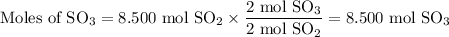
From O₂ :
The molar ratio of SO₂:O₂ is 2 mol O₂:1 mol O₂.
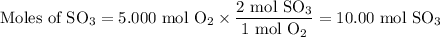
The limiting reactant is SO₂ because it gives the smaller amount of SO₃.
(c) Calculate the theoretical yield of SO₃.
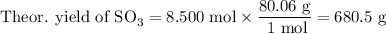
2. Calculate the percent yield of SO₃