Answer:
4
Step-by-step explanation:
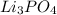 is an ionic crystal rather than a molecule. An ionic crystal dissolved in water dissociates into its cations and anions.
is an ionic crystal rather than a molecule. An ionic crystal dissolved in water dissociates into its cations and anions.
Lithium phosphate consists of two ions. Firstly, the metallic component, lithium, is a positively charged ion in this salt, also known as a cation.
The remaining part, phosphate, is an anion. When lithium phosphate is dissolved in water, the following process takes place:
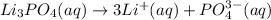
Notice that for 1 formula unit of lithium phosphate, a total of 3 units of lithium cations and 1 unit of phosphate anions are produced.