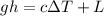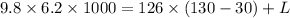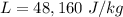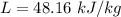Answer:
Step-by-step explanation:
Given
height
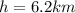
Initial temperature
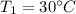
Specific heat of lead
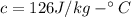
Melting Point of Lead
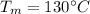
Here Potential Energy is converted to heat energy to melt the lead ball
Sphere ball will first will be heated to
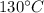 then it starts melting
then it starts melting
thus
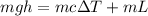
where
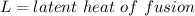
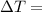 change in Temperature
change in Temperature