Answer:
Mole fraction of methanol will be closest to 4.
Step-by-step explanation:
Given, Mass of methanol = 128 g
Molar mass of methanol = 32.04 g/mol
The formula for the calculation of moles is shown below:
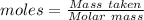
Thus,
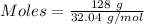
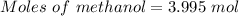
Given, Mass of water = 108 g
Molar mass of water = 18.0153 g/mol
The formula for the calculation of moles is shown below:
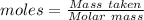
Thus,
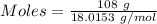
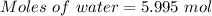
So, according to definition of mole fraction:
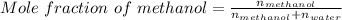
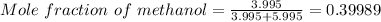
Mole fraction of methanol will be closest to 4.