Answer:
P=133.71mmHg
Step-by-step explanation:
the standard free energy of formation for liquid ethanol is -174/9 kj/mol and that for gaseous ethanol is -168.6 kj/mol. calculate the vapour pressure of ethanol at
assumption:
is that temperature is at 25C, at standard pressure of 1bar(750mmHg)
ethanol is an ideal gas
The free energy of ethanol liquid does not vary with pressure,
C2H5OH(l)⟶C2H5OH(g)
free energy of formation on the reactant side is -174.9 kj/mol
fro the product side is -168.6 kj
∅Gvap-∅G(l)=-168.6kj/mol-(-174.9kj/mol)
+6.3kj/mol
∅G=∅Gvap+RTlnK-∅Gliq
∅G=0
0=+6.3kj/mol+8.314Jk/mol/k(298)InK
-6.3/(RT)=Lnk
taking the exponential of both sides
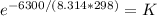
0.178=k
k=p/
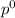
P^0=refers to the pressure of ethanol vapour at its standard state
partial pressure , which is 750 mmHg
P=0.178*750
P=133.71mmHg