Step-by-step explanation:
An alpha particles is basically a helium nucleus and it contains 2 protons and 2 neutrons.
Symbol of an alpha particle is
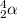 .
.
A beta particle is a particle with a negatively charged electron. Symbol of a beta particle is
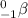 .
.
According to the given question, the decay series chart along with reaction equation are as follows.
(a) Lead-214 changes to bismuth-214.
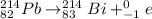
(b) Bismuth-214 changes to polonium-214.
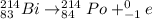
(c) Polonium-214 changes to lead-210.
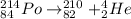
Therefore, we can conclude that proper order of nuclear particle emission is beta, beta, alpha.