Answer:
I recently answered this question. The response I submitted is included below. I beleive my answer should be correct.
Step-by-step explanation:
Question 1:
C: 48.38g(1mol/12g) = 4.0317
H: 8.12g(1mol/1.01g) = 8.12
O: 53.5g(1mol/16g) = 3.34375
Divide by the smallest amount (3.34375)
C = 4.0317/3.34375 = 1.206 = 1
H = 8.12/3.34375 = 2.42 = 2
O = 3.34375/3.34375 = 1
Empirical formula = CH2O
Question 2:
Molecular formula = n(empirical formula)
n = molar mass (compound)/molar mass (empirical)
Empirical formula: CH2O
Molar mass of CH2O = 12 + 2x1 + 16 = 30 g/mol
Molar mass of compound: 180.15 g/mol
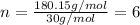
Molecular formula = C6H12O6