Answer:
Option B) KOH has the highest pH and the highest [OH-] concentration.
Step-by-step explanation:
The basicity of a substance increases as the dissociation coefficient increases. Generally, a strong base strongly ionizes in water according to the reaction:
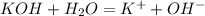
Hence, from the table, KOH is a strong base.