Answer:
4.58g
Step-by-step explanation:
1.) What is the molar mass of CaCI2?
= 110.98 g/mol
2.) Find the mols
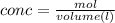
conc × volume= mol
0.125 M x 0.33 L = 0.04125mol ( 330mL was converted to "L" by dividing 330 by 1000)
3.) The mass(g)
g= mol x atomic weight
g= 110.98g/mol × 0.04125
= 4.58 g
# NOTE M = mol/L