- When a magnesium is heated, it burns in air with a dazzling white flame to form a white powder called Magnesium oxide.
- Magnesium + oxygen → Magnesium oxide.
- The burning of magnesium in air to form magnesium oxide is an example of a chemical reaction . In this chemical reaction there are two reactants ' magnesium and oxygen ' but only one product ' magnesium oxide '. The properties of the product magnesium oxide are entirely different from those of the reactants magnesium and oxygen.
Balanced Equation –
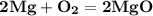
Physical states –
- Magnesium → Mg ( In Solid State)
- Oxygen →
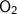 ( Gaseous State)
( Gaseous State)
- Magnesium Oxide → MgO ( In Solid State Or White powder)
_______________________________________