Answer:
83.82 g of magnesium ions.
Step-by-step explanation:
We want to determine the mass of magnesium ions in 155.0 grams of magnesium phosphide (Mg₃P₂).
We can convert from grams of Mg₃P₂ to moles of Mg₃P₂, moles of Mg₃P₂ to moles of Mg, and moles of Mg to grams of Mg.
Find the molecular weight (molar mass) of magnesium phosphide:
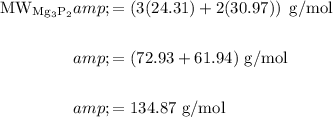
The molecular weight of magnesium is 24.31 g/mol*.
From the chemical formula, we can see that there are three moles of magnesium for every one mole of magnesium phosphide.
Hence, multiply initial amount with known ratios:
![\displaystyle \begin{aligned} &155.0 \text{ g Mg$_3$P$_2$} \cdot \frac{1 \text{ mol Mg$_3$P$_2$}}{134.87\text{ g/mol}} \cdot \frac{3\text{ mol [Mg$^(2+)$]}}{1 \text{ mol Mg$_3$P$_2$}}\cdot \frac{24.31 \text{ g [Mg$^(2+)$]}}{1 \text{ mol [Mg$^(2+)$]}}\\ \\ & = 83.82 \text{ g [Mg$^(2+)$]}\end{aligned}](https://img.qammunity.org/2022/formulas/chemistry/high-school/sybkg83kiegobhev22oyej9gua1y0onb35.png)
In conclusion, there are 83.82 grams of magnesium ions in 155.0 grams of magnesium phosphide.
*Because the magnesium ions in magnesium phosphide have two less electrons, its molecular weight will indeed be lower. However, due to the extremely small size of an electron (1/2000th of a proton), the difference in mass is insignificant.