Answer:
1.6 M.
Step-by-step explanation:
We want to find the molarity of a solution that contains 114.4 g NaCl in a 1.2 L solution.
Recall that molarity is given by:
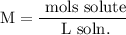
Convert 114.4 g NaCl to mol NaCl using its molecular weight:
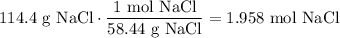
Therefore, the molarity of the solution will be:
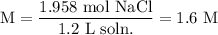
In conclusion, the molarity of the solution is 1.6 M.