Answer:
Neutrons have no charge and are the lightest subatomic particle
Step-by-step explanation:
Nucleus of an atom contains three subatomic particles. These are protons, neutrons and electrons.
Mass of a proton is
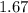 ×
×
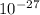 kg.
kg.
Mass of a neutron is
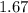 ×
×
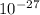 kg.
kg.
Mass of an electron is
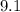 ×
×
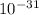 kg.
kg.
Also, protons are positively charged, neutrons have no charge and electrons are negatively charged.
Therefore, we can conclude that the mass of a neutron nearly equals the mass of a proton.