Answer:
0.7 moles of Chlorine gas is required.
Step-by-step explanation:
The reaction is balanced as follows:
2Na +
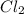 ------> 2NaCl
------> 2NaCl
According to stoichiometry,
2 mole Sodium reacts with 1 mole Chlorine gas
∴1 mole Sodium reacts with 1/2 moles of Chlorine gas
∴1.4 mole sodium reacts with 1.4/2 moles of Chlorine gas
= 0.7 moles of Chlorine gas
So, 0.7 moles of Chlorine gas is required.