Answer:
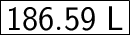
Step-by-step explanation:
Given that:
No. of moles = 8.33 mol
At STP, 1 mol. of all gases has a molar value of 22.4 dm³/mol
So,
Volume of 8.33 mol of N₂ gas:
= no. of moles x molar value
= 8.33 x 22.4
= 186.59 dm³
Since 1 dm³ = 1 L
So,
Volume = 186.59 L
![\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/english/college/eq413d752mwtrwwenrzwldxt4w1olmf1b3.png)