Answer:
1.07 × 10²⁴ carbon atoms.
Step-by-step explanation:
We are given that a beaker contains 44.7 mL of butane and we want to determine how many carbon atoms the liquid contains.
To convert from mL to atoms of C, we can first convert mL to grams, grams to moles, moles to molecules, and molecules to C atoms.
Using its density, determine the amount of grams of butane is in the beaker:
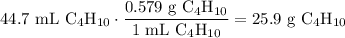
Find the molar mass of butane:

Convert from grams to moles:
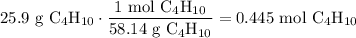
Convert from moles to molecules:
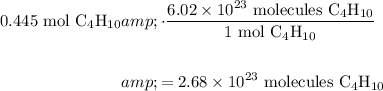
And because there are (exactly) four carbon atoms per butane molecule:
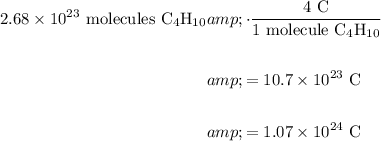
In conclusion, there are 1.07 × 10²⁴ carbon atoms in a beaker containing 44.7 mL of butane.