Answer:
E - Atomic mass is calculated by weighted atomic average using all the isotope data available.
G - Mass number is equal to the sum of protons and electrons in an atom.
Step-by-step explanation:
Take an element
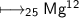
- Mass no is 25 and atomic no is 12.