Answer:

Step-by-step explanation:
We are asked to find how many moles are in a sample of copper (Cu) with a mass of 7.40 grams,
We will convert grams to moles using the molar mass. This is the mass of 1 mole of a substance. These values are found on the Periodic Table because they are equivalent to the atomic masses, but the units are grams per mole instead of atomic mass units.
Look up copper's molar mass.
We will convert using dimensional, so we must set up a ratio.
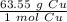
We are converting 7.40 grams of copper to moles, so we multiply by this value.
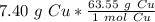
Flip the ratio so the units of grams of copper cancel.
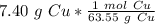
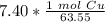
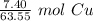
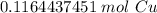
The original measurement of grams (7.40) has 3 significant figures, so our answer must have the same. For the number we found, that is the thousandth place. The 4 in the ten-thousandth place tells us to leave the 6 in the thousandth place.
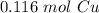
7.40 grams of copper contains approximately 0.116 moles of copper.