Answer:

Step-by-step explanation:
We are asked to find how many atoms are in 6.3 moles of zinc (Zn). We will convert moles to atoms using Avogadro's Number or 6.022 × 10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance.
In this problem, the particles are atoms of zinc. Therefore, there are 6.022 ×10²³ atoms of zinc in 1 mole of zinc.
We will convert using dimensional analysis, so set up a ratio using Avogadro's Number and the underlined information.
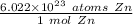
We are converting 6.3 moles of zinc, so we multiply the ratio by this value.
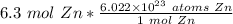
The units of moles of zinc cancel out.
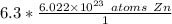
A fraction with a denominator of 1 is equal to the numerator. We can disregard the denominator of 1.
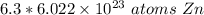
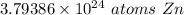
The original measurement of moles has 2 significant figures, so our answer must have the same. For the number we calculated that is the tenths place. The 9 in the hundredth place tells us to round the 7 in the tenths place up to an 8.
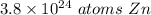
6.3 moles of zinc contains approximately 3.8 ×10²⁴ atoms of zinc.