Answer:
At standard room temperature,
![[{\rm OH^(-)] \approx 2.5 * 10^(-7)\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/high-school/6dm66pmep6f1rhx7w3gswll43lljmhsgv2.png) when
when
![[{\rm H^(+)] = 4.0 * 10^(-8)\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/high-school/2nro3hg9c7esetpqk1c9mpj4hpoxa47qy9.png) .
.
Step-by-step explanation:
The following equilibrium goes on in water:
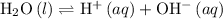 .
.
The forward reaction is known as the self-ionization of water. The ionization constant of water,
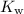 , gives the equilibrium position of this reaction:
, gives the equilibrium position of this reaction:
![K_(\rm w) = [{\rm H^(+)] \cdot [{\rm OH^(-)}]](https://img.qammunity.org/2022/formulas/chemistry/high-school/lzcxjz80ll0vckgltz2ohzywyuwn7za00a.png) .
.
At standard room temperature (
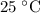 ),
),
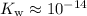 . Also,
. Also,
![[{\rm H^(+)}] = 4.0 * 10^(-8)\; \rm mol \cdot L^(-1)](https://img.qammunity.org/2022/formulas/chemistry/high-school/c86lfnef9ychlkigb3zoc49wqwpb0ul77i.png) . Substitute both values into the equation and solve for
. Substitute both values into the equation and solve for
![[{\rm OH^(-)}]](https://img.qammunity.org/2022/formulas/chemistry/high-school/cfrqs57mz4rv0dtivvxz907hummj522g0j.png) .
.
![\begin{aligned} {[}{\rm OH^(-)}{]} &= \frac{K_(\rm w)}{[{\rm H^(+)}]} \\ &\approx (10^(-14))/(4.0 * 10^(-8)) = 2.5 * 10^(-7)\end{aligned}](https://img.qammunity.org/2022/formulas/chemistry/high-school/a4zej7ilg2241b869eicm0p4fazzkwh7bd.png) .
.
In other words, in an aqueous solution at standard room temperature,
![[{\rm OH^(-)] \approx 2.5 * 10^(-7)\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/high-school/6dm66pmep6f1rhx7w3gswll43lljmhsgv2.png) when
when
![[{\rm H^(+)] = 4.0 * 10^(-8)\; \rm M](https://img.qammunity.org/2022/formulas/chemistry/high-school/2nro3hg9c7esetpqk1c9mpj4hpoxa47qy9.png) .
.