Answer:

Step-by-step explanation:
We are asked to calculate the density of gold. The density of a substance is its mass per unit volume. It is calculated using the following formula.
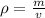
The mass of the sample is 96.5 grams. The volume of the sample is 5.00 cubic centimeters.
Substitute the values into the formula.
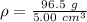
Divide.
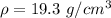
The density of gold is 19.3 grams per cubic centimeter.