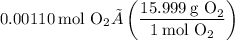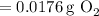Step-by-step explanation:
The chemical reaction that describes the combustion of methane is
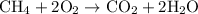
We need to convert the mass of methane to moles:
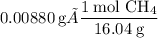
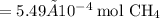
Now use the molar ratios to determine the amount of oxygen used during the combustion:
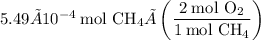
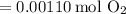
Converting this to grams, we find that the mass of oxygen is