Answer:
which formula can be used to calculate the molar mass of hydrogen peroxide (H2O2)?
» d.2 x molar mass of H + 2 x molar mass of O
Step-by-step explanation:
Since we've two hydrogen atoms and two oxygen atoms, we multiply each mass by 2
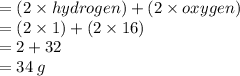
Which of the following statements best defines the actual yield of a reaction?
» d.The ratio of measured yield over stoichiometric yield