Answer:
1.41 x 10 ^4
Step-by-step explanation:
change atoms to mol then to gram
5 x 10^25 atoms of AgNO3
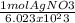
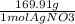
= 141.05g x 10^2 move the decimal over if you are doing sig fig 1.41 x 10^4
change atoms to mol using Avogadro's number 6.023 x 10^23 then mol to gram using the molar mass of AgNO3.