Answer:
5.48 x 10´23 atoms
Step-by-step explanation:
Use PV = nRT
V = 20 L
R= constant = 0,08205746 L atm / (K mol)
stp means stantdard temperature and pression
T = 273 K
P = 0.987 atm
n = PV/RT = (0.987 atm) x (20 L) = 0.88 moles
(0,082 Latm/(K mol)x 273 K
0.88 moles x
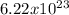 molecules = 5.48 x 10´23 atoms
molecules = 5.48 x 10´23 atoms