Answer:
Ksp = [ Cu+² ] [ OH-] ²
molar mass Cu(oH )2 ==> M= 63.546 (1) + 16 (2) + 1 (2) = 97.546 g/mol
Ksp = [ Cu+² ] [ OH-] ²
Ksp [ cu (OH)2 ] = 2.2 × 10-²⁰
|__________|___Cu+² __|_2OH-____|
|Initial concentration(M)|___0__|_0______|
|Change in concentration(M)|_+S |__+2S__|
|Equilibrium concentration(M)|_S _|2S___|
Ksp = [ Cu+² ] [ OH-] ²
2.2 ×10-²⁰ = (S)(2S)²= 4S³
![s = \sqrt[3]{ \frac{2.2 * {10}^( - 20) }{4} } = 1.8 * {10}^( - 7)](https://img.qammunity.org/2022/formulas/chemistry/college/tx8pc0ewlvba8qxbr0lvssci6lxhleqjm0.png)
S = 1.8 × 10-⁷ M
The molar solubility of Cu(OH)2 is 1.8 × 10-⁷ M
Solubility of Cu (OH)2 =
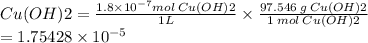
Solubility of Cu (OH)2 = 1.75428 × 10 -⁵ g/ L
I hope I helped you^_^