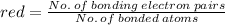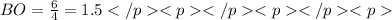1. Sulfur dioxide, or SO2 , has two resonance structures which contribute equally to the overall hybrid structure of the molecule. However, a third Lewis structure can be drawn for SO2 which is more stable in theory, but doesn't quite match experimental data.
2.