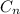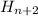Answer:
Option D
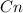
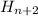
Step-by-step explanation:
Alkanes have nothing but carbon and hydrogen bonds, with no double or triple carbon bonds. Each carbon in the chain will have a unit of CH2, with the other 2 carbon bonds connected to 2 neighboring carbons. The terminal carbons will have 3 H's each.
So an alkane will have multiple (CH2) groups plus 2 CH3 groups on either end. If there are n Carbons, there will be 2n Hydrogens plus 2 more for the end carbons.