Solution :
Cd(s) ---------------------->
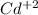 (aq) + 2
(aq) + 2
 ,
,
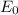 = 0.34 v
= 0.34 v
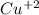 (aq) + 2
(aq) + 2
 ------------> Cu (s) ,
------------> Cu (s) ,
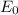 = -0.04 v
= -0.04 v
----------------------------------------------------------------------------------------------
Cd(s) +
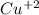 (aq) ------------->
(aq) ------------->
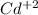 (aq) + Cu (s) ,
(aq) + Cu (s) ,
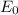 = 0.30 v
= 0.30 v
The cell potential is defined as the measure of
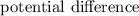 between the
between the
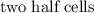 of an electrochemical cell.
of an electrochemical cell.