Answer:
The wavelength of the photon that would have the same momentum as the electron is 202.2180996 nm
Step-by-step explanation:
The velocity of the electron, v = 3.6 × 10³ m/s
The momentum of an electron,
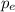 = m × v
= m × v
Where;
v = The mass of the electron = 9.109 × 10⁻³¹ kg
∴
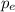 = 9.109 × 10⁻³¹ kg × 3.6 × 10³ m/s = 3.27924 × 10⁻²⁷ kg·m/s
= 9.109 × 10⁻³¹ kg × 3.6 × 10³ m/s = 3.27924 × 10⁻²⁷ kg·m/s
According to the de Broglie equation, the momentum of a photon, p, is given as follows;
p = h/λ
Where;
h = 6.63 × 10⁻³⁴ J·s
λ = The wavelength of the photon
∴ λ = h/p
According to the question, we have;
p =
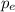 = 3.27924 × 10⁻²⁷ kg·m/s
= 3.27924 × 10⁻²⁷ kg·m/s
∴ λ = 6.63 × 10⁻³⁴ J·s/(3.27924 × 10⁻²⁷ kg·m/s) = 2.02180993 × 10⁻⁷ m
The wavelength of the photon, λ = 2.02180993 × 10⁻⁷ m = 202.2180993 × 10⁻⁹ m = 202.2180993 nm.