Answer:
Step-by-step explanation:
The best way to explain this is to use an example
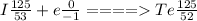
You have to understand what happened. A electron was shot into the nucleus of the Iodine. That electron change the entire composition of the nucleus resulting in 52 protons. The mass remained the same (125) but the nucleus was altered. The chemical became 125 52 Tellurium. But what is important is that it takes a tremendous amount of energy to disrupt a nucleus, and a new chemical is born from that disruption.