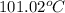Answer: The boiling point of the solution is
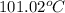
Step-by-step explanation:
We are given:
3.5 % (by weight) NaCl
This means that 3.5 g of NaCl is present in 100 g of solution
Mass of solvent = Mass of solution - Mass of solute
Mass of solvent (water) = (100 - 3.5) g = 96.5 g
Elevation in the boiling point is defined as the difference between the boiling point of the solution and the boiling point of the pure solvent.
The expression for the calculation of elevation in boiling point is:
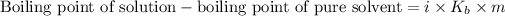
OR
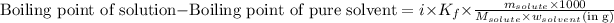 ......(1)
......(1)
where,
Boiling point of pure solvent (water) =
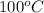
Boiling point of solution = ?
i = Vant Hoff factor = 2 (for NaCl)
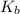 = Boiling point elevation constant =
= Boiling point elevation constant =
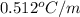
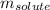 = Given mass of solute (NaCl) = 3.5 g
= Given mass of solute (NaCl) = 3.5 g
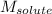 = Molar mass of solute (NaCl) = 36.5 g/mol
= Molar mass of solute (NaCl) = 36.5 g/mol
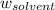 = Mass of solvent (water) = 96.5 g
= Mass of solvent (water) = 96.5 g
Putting values in equation 1, we get:
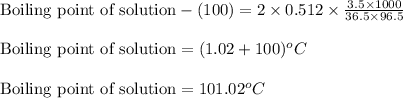
Hence, the boiling point of the solution is