Answer:

Step-by-step explanation:
We are asked to find the new volume of a gas after a change in temperature. We will use Charles's Law, which states the volume of a gas is directly proportional to the temperature. The formula for this law is:
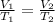
The gas starts at 100 degrees Celsius and a volume of 33.5 liters. Substitute these values into the formula.
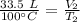
The gas is cooled to 25 degrees Celsius, but the volume is unknown.
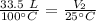
We want to find the volume of the gas after it is cooled. We must isolate the variable V₂. It is being divided by 25 degrees Celsius and the inverse of division is multiplication. Multiply both sides of the equation by 25 °C.
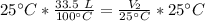
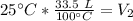
The units of degrees Celsius cancel.
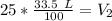
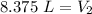
The original measurements have 3 significant figures, so our answer must have the same. For the number we found, that is the hundredth place. The 5 in the thousandths place tells us to round the 7 up to an 8.
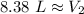
The new volume after the gas is cooled is approximately 8.38 liters.