Answer:
See detailed explanation.
Step-by-step explanation:
Hey there!
In this case, according to the given information, it turns out possible for us to solve this problem by firstly calculating the moles of each element, assuming those percentages are masses, so that we divide by their molar masses:
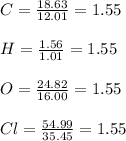
Then, we divide all of them by 1.55 to realize the empirical formula is:
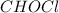
Whose molar mass is 64.47 g/mol, and therefore, since the molar mass of these two is the same, we infer the molecular formula is also CHOCl.
The Lewis structure is shown on the attached document, whereas, the central atom is C and it does complete its octet as well as both O and Cl.
Regards!