Answer:
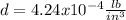
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to set the equation for the calculation of density and mass divided by volume:
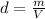
Thus, we can find the mass of the unknown by subtracting the total mass of the liquid to the mass of the flask and the liquid:
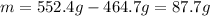
So that we are now able to calculate the density in g/mL first:
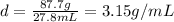
Now, we proceed to the conversion to lb/in³ by using the following setup:
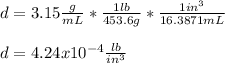
Regards!