Answer:

Step-by-step explanation:
Hello there!
In this case, according to the given description, it turns out possible for us to set up the required chemical reaction as follows:
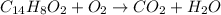
However, it turns out mandatory to balance it according to the law of conservation of mass, which states that the atoms must the same on the reactants side as on the products side; thus, we proceed as follows:

In order to have 14 C atoms, 8 H atoms and 32 O atoms on both sides of the chemical equation.
Regards!