Answer:
2.25 M
Step-by-step explanation:
The reaction that takes place is:
- 2KI + Pb(NO₂)₃ → PbI₂ + 2KNO₃
First we calculate how many potassium iodide moles reacted, using the given volume and concentration:
- 15.00 mL * 3.00 M = 45 mmol KI
Then we convert 45 mmoles of KI into mmoles of Pb(NO₂)₃, using the stoichiometric coefficients of the balanced reaction:
- 45 mmol KI *
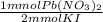 = 22.5 mmol Pb(NO₂)₃
= 22.5 mmol Pb(NO₂)₃
Finally we calculate the molarity of the Pb(NO₂)₃ solution, using the calculated number of moles and given volume:
- 22.5 mmol Pb(NO₂)₃ / 10.00 mL = 2.25 M