Answer:
C. T>617 K
Step-by-step explanation:
We are given that
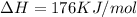
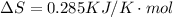
We have to find the temperature at which the reaction is spontaneous.
When
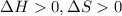
Therefore, the reaction is spontaneous at certain range of temperature.
Option A is not true.
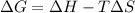
When
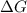 is negative, then the reaction is spontaneous.
is negative, then the reaction is spontaneous.
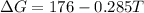
When T<50
Suppose T=49 K
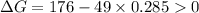
Therefore,
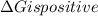 .Hence, the reaction is not spontaneous.
.Hence, the reaction is not spontaneous.
Option B is wrong.
C.T>617K
Suppose T=618 K
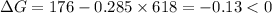
Therefore,
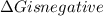 .Hence, the reaction is spontaneous.
.Hence, the reaction is spontaneous.
So, option C is true.
D.T<617 K
Suppose T=616 K
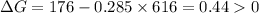
Therefore,
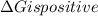 .Hence, the reaction is not spontaneous.
.Hence, the reaction is not spontaneous.
So, option D is not true.