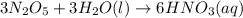Answer:
For 1: The product is phosphoric acid and the solution is acidic in nature.
For 2: The product is sodium hydroxide and the solution is basic in nature.
For 3: The product is nitric acid and the solution is acidic in nature.
Step-by-step explanation:
For the given options:
(1): When diphosphorus pentoxide reacts with water, it leads to the formation of phosphoric acid, which makes the solution acidic in nature.
The chemical equation for the reaction follows:
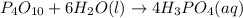
(2): When disodium oxide reacts with water, it leads to the formation of sodium hydroxide, which makes the solution basic in nature.
The chemical equation for the reaction follows:
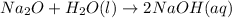
(3): When dinitrogen pentoxide reacts with water, it leads to the formation of nitric acid, which makes the solution acidic in nature.
The chemical equation for the reaction follows: