Answer:
1.99 L
Step-by-step explanation:
Given that,
A reaction produces 3.0 mol of gas, which occupies 1.46 L.
We need to find the volume of the product when 4.1 mol are produced at constant temperature and pressure.
We know that,
PV = nRT
i.e.
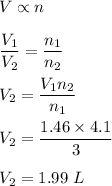
So, the new volume is 1.99 L.