Answer:
1. How many ATOMS of boron are present in 2.20 moles of boron trifluoride? atoms of boron.
2. How many MOLES of fluorine are present in of boron trifluoride? moles of fluorine.
Step-by-step explanation:
The molecular formula of boron trifluoride is
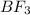 .
.
So, one mole of boron trifluoride has one mole of boron atoms.
1. The number of boron atoms in 2.20 moles of boron trifluoride is 2.20 moles.
The number of atoms in 2.20 moles of boron is:
One mole of boron has ----
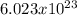 atoms.
atoms.
Then, 2.20 moles of boron has
-
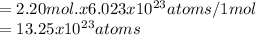
2. Calculate the number of moles of BF3 in 5.35*1022 molecules.
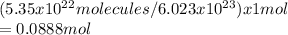
One mole of boron trifluoride has three moles of fluorine atoms.
Hence, 0.0888moles of BF3 has 3x0.0888mol of fluorine atoms.
=0.266mol of fluorine atoms.