Answer:
a. 164°F
b.
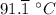
c.
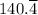 kJ
kJ
Step-by-step explanation:
The starting temperature of the water, T₁ = 48F
The temperature at which the water boils, T₂ = 212°F
a. The difference between the initial and the boiling water temperature, ΔT = T₂ - T₁
Therefore;
ΔT = 212°F - 48°F = 164°F
The temperature by which he temperature must be raised, ΔT = 164°F
b. 48°F = ((48 - 32)×5/9)°C = (80/9)°C =
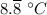
212°F = ((212 - 32)×5/9)°C = 100°C
∴ ΔT = 100°C -
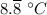 =
=
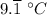
c. The heat capacity of the water = The heat required to increase four quartz of water by 1 °C = 15.8 kJ
∴ The heat required to raise four quartz of water by
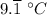 , ΔQ = 15.8 kJ/°C ×
, ΔQ = 15.8 kJ/°C ×
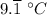 =
=
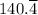 kJ.
kJ.